Blog & News
New Brief Examines Flu Vaccine Patterns as a Proxy for COVID – Anticipating and Addressing Coronavirus Vaccination Campaign Challenges at the National and State Level
January 6, 2021:Within just a year, the U.S. saw both the devastating arrival of the novel coronavirus (COVID-19) as well as the rapid innovation by researchers and scientists that produced multiple vaccines in response to this disease. In the final month of 2020, the U.S. Food and Drug Administration’s (FDA) approval of two such vaccinations developed by Pfizer-BioNTech and Moderna marked a significant step in the battle toward preventing and controlling the spread of the pandemic.
Across the United States, the rollout of the vaccine to health care and essential workers is already underway; however, once this first tier of recipients has been vaccinated, states will need to begin subsequent phases of a massive vaccination campaign among their larger resident populations. The ultimate goal is to reach sufficient levels of COVID vaccination in order to achieve protective population immunity, or “herd immunity.”
But efforts toward that aim may be hampered by various hurdles, including vaccine skepticism and a U.S. health care system that typically leaves large segments of the population underserved.
While public health officials design and implement their vaccination plans, the nation’s experiences with other vaccine campaigns can provide insights as to the specific challenges faced by states in attaining their targets for coronavirus immunization. A new SHADAC report funded by the California Health Care Foundation (CHCF) and authored by SHADAC researchers Colin Planalp, MPA, and Robert Hest, MPP, uses data from the U.S. Centers for Disease Control and Prevention’s (CDC) Behavioral Risk Factor Surveillance System (BRFSS) survey to examine flu vaccination rates across multiple years for U.S. adults (age 18 and older) across the 50 states and the District of Columbia as a proxy to identify population subgroups that may be harder to reach with a COVID-19 vaccine. The brief also provides an analysis of several demographic categories, including indicators of health and health care access, for the U.S. and California, specifically.1
Key Findings – Vaccination Landscape
A number of experts have previously agreed that in the U.S., a minimum threshold of approximately 70% immunity among the national population must be reached in order to bring the COVID-19 epidemic under control.2 Even that target of 70% may be insufficient, and some experts such as National Institute of Allergies and Infectious Diseases (NIAID) Director Dr. Anthony Fauci and other prominent epidemiologists have more recently recommended shifting the goal to 80% or even higher. However, multiple surveys have found that while public willingness currently sits at its highest reported rate to date, it still often falls short of those rates.3 While it is unclear how survey results may translate into vaccine take-up, when looking at analog flu vaccine rate several barriers to achieving these ambitious immunity goals become apparent.
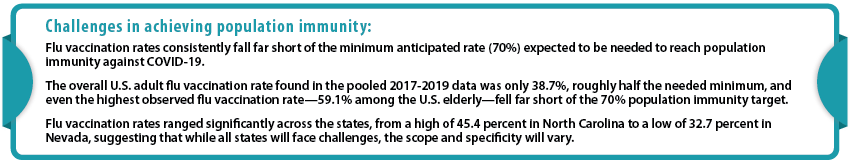
Key Findings – Vaccination Trends for U.S. and California
While it is important to consider national and state data, it is also vital to remember that looking only at estimates on these levels can mask significant differences and disparities across subpopulations that are likely to require targeted efforts in a vaccination campaign. Achieving population immunity against COVID-19 across both the broader population and particular communities is important for health equity. It also is important to protect against continued smaller-scale outbreaks of COVID-19 for years to come, such as the occurrences experienced by multiple states in recent years from measles—a disease that was once considered to be eliminated from the U.S.4 To that end, the issue brief looked across subgroups in the U.S. and California, to identify which groups and may be hardest to reach with a COVID-19 vaccine.

Looking Ahead
While not a perfect analog for COVID-19, the flu vaccination rates presented in this brief are probably the best available proxy to predict the challenges the U.S. and states face in implementing a widespread COVID-19 vaccination campaign following the recent approval of the first such vaccines in the nation, with others anticipated to follow.5

Understanding these challenges and subpopulation differences can help states, such as California, identify which groups may be hardest to reach with a COVID-19 vaccine as well as craft strategies and guide outreach efforts to ensure the greatest equity and effectiveness in vaccinating the populations of states and the U.S. against the coronavirus.
This work is supported by the California Health Care Foundation.
Notes.
1 To enhance the ability to reliably measure vaccination rates for relatively small subgroups, SHADAC authors combined three years of data from the 2017-2019 BRFSS surveys, and estimates in the report represent an average of this time period.
2 Mayo Clinic. (n.d.). Herd immunity and COVID-19 (coronavirus): What you need to know. https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/herd-immunity-and-coronavirus/art-20486808
Kwok, K.O., Lai, F., Wei, W.I., Shan Wong, S.Y., & Tang, J.W.T. (2020). Herd immunity – estimating the level required to halt the COVID-19 epidemics in affected countries. Journal of Infection, 80(6), e32–e33. doi: 10.1016/j.jinf.2020.03.027
Moore, K.A., Lipsitch, M., Barry, J.M., & Osterholm, M.T. (2020, April 30). COVID-19: The CIDRAP Viewpoint. Center for Infectious Disease Research and Policy (CIDRAP); University of Minnesota. https://printabletemplates.com/cidrap-covid19-viewpoint/
3 MacNeil, Jr., D.G. (2020, December 24). How much herd immunity is enough? The New York Times. https://www.nytimes.com/2020/12/24/health/herd-immunity-covid-coronavirus.html
4 Centers for Disease Control and Prevention (CDC). (2020, December 2). Measles cases and outbreaks. https://www.cdc.gov/measles/cases-outbreaks.html
5 Centers for Disease Control and Prevention (CDC). (2020, December 28). Different COVID-19 vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html










